Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Sasaki, Yuji; Morita, Keisuke; Kitatsuji, Yoshihiro; Ito, Keisuke*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 28(2), p.121 - 131, 2021/00
Times Cited Count:2 Percentile:14.88(Chemistry, Multidisciplinary)High concentration of Cs is present in high-level radioactive waste. It is well-known that Cs is an alkali element and difficult to extract completely into an organic phase. Crown ether compounds are widely available for Cs extractants; DtBuDB18C6 (di- -butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as
-butyl-dibenzo-18crown6), was used in this study. Organic solvents used for the industrial applications, such as  -dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios (
-dodecane and 1-octanol, have low solubility concerning the compound; other solvents were employed and tested. In this study, ketone-, ether-, and ester-type solvents showed high solubility for DtBuDB18C6 and DtBuDB18C6, when dissolved in ketones and alcohols, exhibited relatively high Cs distribution ratios ( (Cs)), closely to 10.
(Cs)), closely to 10.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Sasaki, Yuji; Saeki, Morihisa*; Yoshizuka, Kazuharu*
Solvent Extraction Research and Development, Japan, 26(1), p.21 - 34, 2019/06
Times Cited Count:6 Percentile:26.61(Chemistry, Multidisciplinary)Three tridentate extractants and three masking reagents including O, N, and S donors have been developed and their properties are compared and discussed. The extractants are termed as tetraoctyl-diglycolamide (TODGA), methylimino-dioctylacetamide (MIDOA) and tetraoctyl-thiodiglycolamide (TDGA(C8)) and masking agents have the same central frame but with short alkyl chain. The results of the present study indicate that TODGA can extract mainly hard acid metals belonging groups 2-4,13-15 in periodic table, MIDOA can extract soft acid metals and oxyanions (groups 5-10, 16), and TDGA can extract soft acid metals (groups 10-11). Some spectrophotometric studies (UV, IR, and NMR) indicate the stoichiometry and effect of donor atoms for metal-complexation. The Hf values, the heat generation during complex formation, obtained by chemical calculation by DFT theory show the reverse-correlation with their extraction ability.
 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:14.02(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
Hatakeyama, Mizuki*; Nishiyama, Yoshio*; Nagatani, Hirohisa*; Okamura, Hiroyuki; Imura, Hisanori*
Solvent Extraction Research and Development, Japan, 25(2), p.79 - 89, 2018/00
Times Cited Count:12 Percentile:42.69(Chemistry, Multidisciplinary)The synergistic extraction of trivalent lanthanide ions (Ln(III)) with benzoylacetone (Hba) and trioctyphosphine oxide (TOPO) in an ionic liquid (IL), 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide ([Bmim][Tf N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba)
N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba) (TOPO)
(TOPO)
 or Ln(ba)(TOPO)
or Ln(ba)(TOPO)
 with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
 is the most stable complex in the IL.
is the most stable complex in the IL.
Sasaki, Yuji; Morita, Keisuke; Suzuki, Shinichi; Shiwaku, Hideaki; Ito, Keisuke; Takahashi, Yuya*; Kaneko, Masaaki*
Solvent Extraction Research and Development, Japan, 24(2), p.113 - 122, 2017/06
The solvent extraction of Se, Zr, Pd, and Cs from nitric acid into 1-octanol (OC) and dodecane has been performed. These elements include long-lived radionuclides in spent nuclear fuels, so a simple separation method is indispensable for the development of the treatment of high-level liquid radioactive waste. It was found that Se can be extracted using phenylenediamine, Zr can be extracted using tetraoctyl diglycolamide and di-2-ethylhexyl phosphoric acid, and Pd can be extracted using (methylimino)bis(dioctylacetamide) and hexaoctylnitrilotriacetamide. These elements can be recovered in over 90% yield by these extractants from nitric acid into OC. A distribution ratio of Cs of greater than 1 can be obtained using di-t-butyldibenzo-18-crown-6. It is clear that 90% recovery of Cs can be achieved using an extraction solvent with ten times the volume of the aqueous phase.
Sugita, Tsuyoshi; Fujiwara, Iori*; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika; Shimojo, Kojiro
Solvent Extraction Research and Development, Japan, 24(2), p.61 - 69, 2017/05
We investigated an influence of amide group in diglycolamic acid-type extractants on extraction property of metal ions. The extraction characteristics of  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of
DGAA), with a secondary amide group, for 56 metal ions have been investigated, and compared with those of  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. Compared with DODGAA, C DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C
DGAA has a poor extraction performance and separation ability for rare-earth metal ions, except for Sc(III). However, C DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C
DGAA tended to provide better extraction for relatively small-sized metal ions than DODGAA. In addition, it was found that C DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
DGAA enables the selective removal of Hg(II) from aqueous solutions containing various divalent metal ions.
Shimojo, Kojiro; Fujiwara, Iori*; Fujisawa, Kiyoshi*; Okamura, Hiroyuki; Sugita, Tsuyoshi; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 23(2), p.151 - 159, 2016/05
Liquid-liquid extraction of rare-earth (RE) cations has been investigated using  -dodecyldiglycolamic acid (C
-dodecyldiglycolamic acid (C DGAA) with a secondary amide group, and compared with that using
DGAA) with a secondary amide group, and compared with that using  -dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C
-dioctyldiglycolamic acid (DODGAA) with a tertiary amide group. C DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C
DGAA enables quantitative transfer of all RE cations from moderately acidic solution, while being selective toward the heavier RE cations, and performs better than typical carboxylic-acid-type extractants. However, C DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE
DGAA provides low extraction performance and separation ability for RE cations compared with DODGAA because of the weaker basicity of the amide oxygen. Slope analysis demonstrated that RE transfer with C
transfer with C DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C
DGAA proceeded through a proton-exchange reaction, forming a 1:3 complex, RE(C DGAA)
DGAA) . Structural characterization by X-ray diffraction revealed that three
. Structural characterization by X-ray diffraction revealed that three  -butyldiglycolamic acid (C
-butyldiglycolamic acid (C DGAA) molecules coordinated to the La
DGAA) molecules coordinated to the La central ion in a tridentate fashion and the La
central ion in a tridentate fashion and the La primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
primary coordination sphere consisted of three oxygen atoms from the amide group, three oxygen atoms from the ether group, and three oxygen atoms from the carboxy group.
Sasaki, Yuji; Morita, Keisuke; Shimazaki, Shoma*; Tsubata, Yasuhiro; Ozawa, Masaki*
Solvent Extraction Research and Development, Japan, 23(2), p.161 - 174, 2016/05
We examined the masking effects of 16 water-miscible reagents, on the extraction of Mo, Re, Ru, and Pd. The extractants, methylimino-dioctylacetamide (MIDOA) and hexaoctyl-nitrilotriacetamide (NTAamide(C8)), show significantly high distribution ratios for these metals, were employed in this study. Masking effects were observed as a decrease of distribution ratio with an increase of masking agent concentration in these extraction systems. The results showed that Pd and Ru can be masked by similar reagents including N- or S- donor atoms, which suppressed the extraction into the organic phase. In contrast, distribution ratio of Mo was only slightly masked by the above mentioned reagents. The masking of Mo was achieved using complexing agents having a central N(CH C(P)=O)
C(P)=O) framework that is important for this purpose. A masking agent for Re was not found in this study.
framework that is important for this purpose. A masking agent for Re was not found in this study.
Sasaki, Yuji; Saeki, Morihisa; Sugo, Yumi; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Ohashi, Akira*
Solvent Extraction Research and Development, Japan, 22(1), p.37 - 45, 2015/05
Times Cited Count:14 Percentile:42.85(Chemistry, Multidisciplinary)An extractant, methylimino-bis- -dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis-
-dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis- -dioctylacetamide (IDOA) and methylimino-bis-
-dioctylacetamide (IDOA) and methylimino-bis- -di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower
-di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower  values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA (
values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA ( -tetraoctyl-diglycolamide) and TDGA (
-tetraoctyl-diglycolamide) and TDGA ( -tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N
-tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N  S
S  O and N
O and N  O
O  S, respectively.
S, respectively.
 -di(2-
-di(2-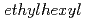 )-2,2-dimethylpropanamide (DEHDMPA) and
)-2,2-dimethylpropanamide (DEHDMPA) and  -di(2-
-di(2-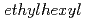 )butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBA
)butanamide (DEHBA) using mixer-settlers in the presence of degradation products of DEHDMPA and DEHBABan, Yasutoshi; Hotoku, Shinobu; Tsubata, Yasuhiro; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction Research and Development, Japan, 22(1), p.47 - 55, 2015/00
Two sets of continuous counter-current experiments using mixer-setters were performed for evaluating extraction properties of  -dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed
-dialkylamides toward U and Pu in the presence of their degradation products. The 1st cycle employed  -di(2-
-di(2-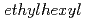 )-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed
)-2,2-dimethylpropanamide (DEHDMPA) for selective extraction of U(VI), and the 2nd cycle employed  -di(2-
-di(2-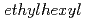 )butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1
)butanamide (DEHDBA) for co-extraction of U(VI) and Pu(IV). Degradation products were added to the organic phase of each cycle. Most of U was effectively extracted by DEHDMPA, and the ratio of U recovered to the U fraction was 99.57%. DEHDMPA barely extracted Pu, and the decontamination factor of U with respect to Pu in the U fraction was 1.1  10
10 . The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
. The raffinate of the 1st cycle was used as the feed in the 2nd cycle, and the residual U and almost all Pu were effectively extracted by DEHBA. The degradation products had no detrimental effects on the two-phase separation and the operation of mixer-settlers.
Nagano, Tetsushi; Mitamura, Hisayoshi; Yamashita, Yuji; Yanase, Nobuyuki; Suzuki, Hideya; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 21(1), p.111 - 117, 2014/00
Simulated electroless nickel plating liquid wastes have been processed by using an emulsion flow extractor of a counter current type with a special focus on influences of dilution of the liquid wastes on the extraction performance. The emulsion flow extractor provides an efficient liquid-liquid extraction by sending solutions without additional stirring or shaking. A solvent used in the present study was Shellsol D70 solution containing LIX84-I as an extractant for nickel and PC88A as an accelerating agent. As a result, it was found that increasing degree of dilution with water resulted in improvement of nickel extractabilities obtained from the emulsion flow experiments with a maximum value of 96% as well as those obtained from batch experiments. Droplet sizes at the lower and the upper sides of emulsion phases, estimated by using high-speed microscope, were 214  36
36  m and 415
m and 415  110
110  m, respectively.
m, respectively.
Akutsu, Kazuhiro*; Suzuki, Shinichi; Kobayashi, Toru; Shiwaku, Hideaki; Okamoto, Yoshihiro; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 20, p.105 - 114, 2013/00
Sasaki, Yuji; Kitatsuji, Yoshihiro; Sugo, Yumi; Tsubata, Yasuhiro; Kimura, Takaumi; Morita, Yasuji
Solvent Extraction Research and Development, Japan, 19, p.51 - 61, 2012/00
Diamides and phosphine oxides having many kinds of the central frames were synthesized and employed in order to see the behavior of actinides(An)(III), (IV), (V) and (VI). Diamides synthesized here have two, three, or four donor atoms of amidic and etheric oxygen (nitrogen or sulfur, instead) atoms in their central frames, namely the extractants can work as bi-, tri-, and tetradentate modes. Di-phosphine di-oxides ((Bis(diphenylphosphoryl)methane (BDPPM) and bis(diphenylphosphoryl)ethylene (BDPPE)), carbamoylmethylphosphine oxide (octylphenyl- ,
, -diisobutyl-carbamoylmethylphosphine oxide (CMPO)) are used as the representative actinide extractants. Metal ions of Eu(III), Th(IV), U(VI), Np(V), Pu(IV) and Am(III) in perchloric acid were extracted into nitrobenzene or n-dodecane. Measurement of
-diisobutyl-carbamoylmethylphosphine oxide (CMPO)) are used as the representative actinide extractants. Metal ions of Eu(III), Th(IV), U(VI), Np(V), Pu(IV) and Am(III) in perchloric acid were extracted into nitrobenzene or n-dodecane. Measurement of  value at the different extractant concentration gives the information on extraction reaction and their extraction ability. From the present work, the best extractant is BDPPM, due to the strong affinity concerning P=O and form of the bidentate mode with six-membered ring chelation. However, this extractant have low solubility in
value at the different extractant concentration gives the information on extraction reaction and their extraction ability. From the present work, the best extractant is BDPPM, due to the strong affinity concerning P=O and form of the bidentate mode with six-membered ring chelation. However, this extractant have low solubility in  -dodecane. Taking the applicatory properties, e.g., high solubility in
-dodecane. Taking the applicatory properties, e.g., high solubility in  -dodecane, easy organic synthesis, gasification by combustion and high actinide extractability, into consideration, diglycolamide (DGA) is a promising extractant for radioactive waste treatment.
-dodecane, easy organic synthesis, gasification by combustion and high actinide extractability, into consideration, diglycolamide (DGA) is a promising extractant for radioactive waste treatment.
 -dialkylacetamide) (IDAA)
-dialkylacetamide) (IDAA)Sasaki, Yuji; Sugo, Yumi; Saeki, Morihisa; Morita, Yasuji; Ohashi, Akira*
Solvent Extraction Research and Development, Japan, 18, p.69 - 74, 2011/00
The new diamide-type extractant, 2,2-(Imino)bis( -dialkylacetamide) (IDAA), was developed for Tc recovery. IDAA has a nitrogen donor atom introduced into the center of backbone and shows the tridentate feature by hybrid donor atoms of two carbonyl oxygen and a nitrogen. 2,2-(Methylimino)bis(
-dialkylacetamide) (IDAA), was developed for Tc recovery. IDAA has a nitrogen donor atom introduced into the center of backbone and shows the tridentate feature by hybrid donor atoms of two carbonyl oxygen and a nitrogen. 2,2-(Methylimino)bis( -Dioctylacetamide) (MIDOA), 2,2-(Methylimino)bis(
-Dioctylacetamide) (MIDOA), 2,2-(Methylimino)bis( -Didodecylacetamide) (MIDDA), and 2,2-(Imino)bis(
-Didodecylacetamide) (MIDDA), and 2,2-(Imino)bis( -Didodecylacetamide) (IDDA), the IDAA derivatives, can be dissolved well in n-dodecane, and are readily used in the solvent extraction. In addition to Tc(VII) and Re(VII), Cr(VI), Mo(VI), W(VI), Pd(II) and Pu(IV) can be extracted from nitric acid to n-dodecane by these extractants, therefore IDAA has high extractability with some oxonium anions. The extraction capacity for Re(VII) was measured and more than 37 mM Re(VII) can be extracted by 0.1 M MIDOA.
-Didodecylacetamide) (IDDA), the IDAA derivatives, can be dissolved well in n-dodecane, and are readily used in the solvent extraction. In addition to Tc(VII) and Re(VII), Cr(VI), Mo(VI), W(VI), Pd(II) and Pu(IV) can be extracted from nitric acid to n-dodecane by these extractants, therefore IDAA has high extractability with some oxonium anions. The extraction capacity for Re(VII) was measured and more than 37 mM Re(VII) can be extracted by 0.1 M MIDOA.
Sasaki, Yuji; Kitatsuji, Yoshihiro; Tsubata, Yasuhiro; Sugo, Yumi; Morita, Yasuji
Solvent Extraction Research and Development, Japan, 18, p.93 - 101, 2011/00
The mutual separation of Am, Cm and lanthanides (Ln) by the hydrophilic and lipophilic ligands present in both aqueous and organic phases were studied. The hydrophilic ligands used here are DTPA, malonamide, amicacid, TEDGA and DOODA (C2) and four kinds of extractants, TODGA, TDdDGA, DOODA (C8) and DOODA (C12). DOODA can extract light lanthanides with higher D values into the organic phase, on the other hand DGA shows strong complexing ability with middle and heavy lanthanides in the aqueous phase. The high separation factor, over 1000, of La and Gd can be seen in the extraction using extractant, TDdDGA, and DOODA(C2) as the masking agent. The separation factors of Am/Cm by DOODA(C8, C12) and DGA alone extractants are 1.51-1.94, the values with over 3 can be attained by the proposed conditions in this work.
Ozawa, Masaki; Suzuki, Shinichi; Takeshita, Kenji*
Solvent Extraction Research and Development, Japan, 17, p.19 - 34, 2010/00
A hydrometallurgical separation technologies by novel solvent extraction (SX), ion exchange chromatography (IXC) and electrolytic extraction techniques are reviewed as separation tools for light PGM (Ru, Rh, Pd), Tc and  -elements in high level liquid wastes of the nuclear fuel cycle. The SX process using N,N-dialkylamide can isolate U(VI) from fission products without Pu(IV) valence control, and extractants with soft-hard hybrid donors (PTA and PDA) and those containing six soft donors (TPEN) show good separation of actinides (III) from lanthanides (III). The catalytic electrolytic extraction (CEE) process utilizing Pd
-elements in high level liquid wastes of the nuclear fuel cycle. The SX process using N,N-dialkylamide can isolate U(VI) from fission products without Pu(IV) valence control, and extractants with soft-hard hybrid donors (PTA and PDA) and those containing six soft donors (TPEN) show good separation of actinides (III) from lanthanides (III). The catalytic electrolytic extraction (CEE) process utilizing Pd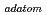 or Rh
or Rh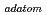 can effectively separate platinum group metals (PGM), Tc and Re by means of controlled under potential deposition (UPD). Some of the basic work on the hydrometallurgical separation of the elements of interest has been carried out through the strategic Advanced (Adv.-) ORIENT Cycle research in Japan.
can effectively separate platinum group metals (PGM), Tc and Re by means of controlled under potential deposition (UPD). Some of the basic work on the hydrometallurgical separation of the elements of interest has been carried out through the strategic Advanced (Adv.-) ORIENT Cycle research in Japan.
Kubota, Fukiko*; Shimobori, Yosuke*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 16, p.142 - 146, 2009/00